Draft:Nuclear Spin
A nuclear spin is a quantum mechanical trait of the nucleus of an atom.
Superficially (or rather: classically) we can imagine an atomic core as a particle orbiting like Earth or a gyroscope around its own orbit.
in quantum mechanics, however, the principle of duality is assumed, which states that particles always also possess wave properties such as a wavelength. When a wave is reflected or rotates on its own axis, interference can occur with itself and this results in only certain states of the spin is possible. This statement is described by quantum numbers. For the nucleus spin, usually the symbol I is used, for the electron, usually the symbol s.
The value of I determines the angular momentum of the particle according to the formula:
Here, ℏ is the reduced constant of Planck.
Values of I
[edit]Each isotope of a nucleus has a specific value of the quantum number I. This number can take values from 0 to 8 in increments of ½. For example, 4He - the alpha particle - has an I = 0 spin, 1H - the proton - has an I = ½ spin, and the niobium isotope 90Nb has an I = 8 which is the highest value found in the periodic table.
The value of the nuclear spin depends on the number of protons (Z) and neutrons (N) in the nucleus.[1] We can distinguish three cases:
- When Z (protons) equals N (neutrons), and I (nuclear spin)= 0. Examples: 4He, 32S
- When Z (protons) equals N (neutrons), but I (nuclear spin) is a positive integer. Examples: 4N: I=1; 10B: I=3
- When Z and N are either both even or both odd, or when they're mixed (one even and one odd). The value of I (nuclear spin) is then a positive half-integer. Examples: 1H: I=½; 23Na: I=3/2
Spin stands
[edit]A nuclear spin can take on 2I+1 spin states. For example, a spin with I=3 can have 7 spin states: |-3>, |-2>, |-1>, |0>, |1>, |2> and |3>. Without an external magnetic field, they decay into the same energy state, meaning that they are degenerate.
In the presence of a magnetic field Bo , Zeeman splitting occurs, and they have different energies. In a magnetic field, a spin will exhibit a axial precession motion, similar to a top in the gravitational field of the Earth and also the Earth itself. The spin states give up that component of the total spin which is in the direction of the magnetic field (the z-component). For example, for the |0> state, the I-spin perpendicular around B, and the z-component of the spin (in the direction of the magnetic field) is zero.
For a proton with I=½ (or an electron with s=½ as shown in the image) there are only 2*½+1=2 spin states, and the energy splitting between them is directly proportional to the size of the magnetic field.[2]
Spectroscopy
[edit]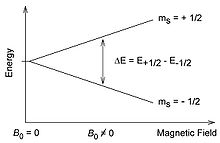
Spin states with I=0 possess only one spin state, making them unsuitable for spectroscopy.
Spin states with a spin of I=½ are very suitable for nuclear magnetic resonance (NMR). Larger spins can also be used, but they don't work as well.
All elements on the periodic table have at least one isotope that can be used for resonance, but it is not always the most abundant one. Especially for elements with atomic number (Z) equal to an even number, the most abundant isotope usually has a spin state of I=0.[1]
References
[edit]- ^ a b "mri-q.com Predicting nuclear spins". Archived from the original on 10 November 2019. Retrieved 29 May 2016.
- ^ "mri-q.com Zeeman splitting". Archived from the original on 10 November 2019. Retrieved 4 March 2025.


