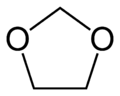
Dioxolane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3-Dioxolane[3] | |||
| Systematic IUPAC name
1,3-Dioxacyclopentane | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.422 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 1166 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6O2 | |||
| Molar mass | 74.08 g/mol | ||
| Density | 1.06 g/cm3 | ||
| Melting point | −95 °C (−139 °F; 178 K) | ||
| Boiling point | 75 °C (167 °F; 348 K) | ||
| Hazards | |||
| GHS labelling:[4] | |||

| |||
| Danger | |||
| H225 | |||
| P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at the 2-position with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals.
As a class of compounds
[edit]Dioxolanes are a group of organic compounds containing the dioxolane ring. Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol.[5]
(+)-cis-Dioxolane is the trivial name for L-(+)-cis-2-methyl-4-trimethylammoniummethyl-1,3-dioxolane iodide which is a muscarinic acetylcholine receptor agonist.
Protecting groups
[edit]Organic compounds containing carbonyl groups sometimes need protection so that they do not undergo reactions during transformations of other functional groups that may be present. A variety of approaches to protection and deprotection of carbonyls[6] including as dioxolanes[7] are known. For example, consider the compound methyl cyclohexanone-4-carboxylate, where lithium aluminium hydride reduction will produce 4-hydroxymethylcyclohexanol. The ester functional group can be reduced without affecting the ketone by protecting the ketone as a ketal. The ketal is produced by acid catalysed reaction with ethylene glycol, the reduction reaction carried out, and the protecting group removed by hydrolysis to produce 4-hydroxymethylcyclohexanone.

NaBArF4 can also be used for deprotection of acetal or ketal-protected carbonyl compounds.[6][7] For example, deprotection of 2-phenyl-1,3-dioxolane to benzaldehyde can be achieved in water in five minutes at 30 °C.[8]
- PhCH(OCH2)2 + H2O PhCHO + HOCH2CH2OH
Natural products
[edit]Neosporol is a natural product that includes a 1,3-dioxolane moiety, and is an isomer of sporol which has a 1,3-dioxane ring.[9] The total synthesis of both compounds has been reported, and each includes a step in which a dioxolane system is formed using trifluoroperacetic acid (TFPAA), prepared by the hydrogen peroxide – urea method.[10][11] This method involves no water, so it gives a completely anhydrous peracid,[12] necessary in this case as the presence of water would lead to unwanted side reactions.[10]
- CF
3COOCOCF
3 + H
2O
2•CO(NH
2)
2 → CF
3COOOH + CF
3COOH + CO(NH
2)
2
In the case of neosporol, a Prilezhaev reaction[13] with trifluoroperacetic acid is used to convert a suitable allyl alcohol precursor to an epoxide, which then undergoes a ring-expansion reaction with a proximate carbonyl functional group to form the dioxolane ring.[10][11]

A similar approach is used in the total synthesis of sporol, with the dioxolane ring later expanded to a dioxane system.[9]
See also
[edit]References
[edit]- ^ 1,3-Dioxolane at Sigma-Aldrich
- ^ formal glycol - PubChem Public Chemical Database
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 145. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ "1,3-Dioxolane". pubchem.ncbi.nlm.nih.gov.
- ^ R. A. Daignault, E. L. Eliel (1973). "2-Cyclohexyloxyethanol (involves acetalisation of cyclohexanone)". Organic Syntheses; Collected Volumes, vol. 5, p. 303.
- ^ a b Greene, Theodora W.; Wuts, Peter G. M. (1999). "Dimethyl acetals". Greene's Protective Groups in Organic Synthesis (3rd ed.). Wiley-Interscience. pp. 297–304, 724–727. ISBN 9780471160199. Archived from the original on December 3, 2016. Retrieved June 20, 2017.
- ^ a b Greene, Theodora W.; Wuts, Peter G. M. (1999). "1,3-Dioxanes, 1,3-Dioxolanes". Greene's Protective Groups in Organic Synthesis (3rd ed.). Wiley-Interscience. pp. 308–322, 724–727. ISBN 9780471160199. Archived from the original on December 7, 2016. Retrieved June 20, 2017.
- ^ Chang, Chih-Ching; Liao, Bei-Sih; Liu, Shiuh-Tzung (2007). "Deprotection of Acetals and Ketals in a Colloidal Suspension Generated by Sodium Tetrakis(3,5-trifluoromethylphenyl)borate in Water". Synlett. 2007 (2): 283–287. doi:10.1055/s-2007-968009.
- ^ a b Pirrung, Michael C.; Morehead, Andrew T.; Young, Bruce G., eds. (2000). "10. Neosporol, Sporol". Part B: Bicyclic and Tricyclic Sesquiterpenes. The Total Synthesis of Natural Products. Vol. 11. John Wiley & Sons. pp. 222–224. ISBN 9780470129630.
- ^ a b c Ziegler, Fredrick E.; Metcalf, Chester A.; Nangia, Ashwini; Schulte, Gayle (1993). "Structure and total synthesis of sporol and neosporol". J. Am. Chem. Soc. 115 (7): 2581–2589. doi:10.1021/ja00060a006.
- ^ a b Caster, Kenneth C.; Rao, A. Somasekar; Mohan, H. Rama; McGrath, Nicholas A.; Brichacek, Matthew (2012). "Trifluoroperacetic Acid". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt254.pub2. ISBN 978-0471936237.
- ^ Cooper, Mark S.; Heaney, Harry; Newbold, Amanda J.; Sanderson, William R. (1990). "Oxidation Reactions Using Urea–Hydrogen Peroxide; A Safe Alternative to Anhydrous Hydrogen Peroxide". Synlett. 1990 (9): 533–535. doi:10.1055/s-1990-21156.
- ^ Hagen, Timothy J. (2007). "Prilezhaev reaction". In Li, Jie Jack; Corey, E. J. (eds.). Name Reactions of Functional Group Transformations. John Wiley & Sons. pp. 274–281. ISBN 9780470176504.



![{\displaystyle {\ce {->[{\ce {NaBAr4}}][{\text{30 °C / 5 min}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3fb42849d6133fe653a7fe5dd019b12e0f6184b5)