5-HT3 receptor
This article may be too technical for most readers to understand. (March 2024) |
The 5-HT3 receptor belongs to the Cys-loop superfamily of ligand-gated ion channels (LGICs) and therefore differs structurally and functionally from all other 5-HT receptors (5-hydroxytryptamine, or serotonin receptors) which are G protein-coupled receptors.[1][2][3] This ion channel is cation-selective and mediates neuronal depolarization and excitation within the central and peripheral nervous systems.[1]
As with other ligand gated ion channels, the 5-HT3 receptor consists of five subunits arranged around a central ion conducting pore, which is permeable to sodium (Na), potassium (K), and calcium (Ca) ions. Binding of the neurotransmitter 5-hydroxytryptamine (serotonin) to the 5-HT3 receptor opens the channel, which, in turn, leads to an excitatory response in neurons. The rapidly activating, desensitizing, inward current is predominantly carried by sodium and potassium ions.[2] 5-HT3 receptors have a negligible permeability to anions.[1] They are most closely related by homology to the nicotinic acetylcholine receptor.
Structure
[edit]The 5-HT3 receptor differs markedly in structure and mechanism from the other 5-HT receptor subtypes, which are all G-protein-coupled. A functional channel may be composed of five identical 5-HT3A subunits (homopentameric) or a mixture of 5-HT3A and one of the other four 5-HT3B,[4][5][6][7] 5-HT3C, 5-HT3D, or 5-HT3E subunits (heteropentameric).[8] It appears that only the 5-HT3A subunits form functional homopentameric channels. All other subunit subtypes must heteropentamerize with 5-HT3A subunits to form functional channels. Additionally, there has not currently been any pharmacological difference found between the heteromeric 5-HT3AC, 5-HT3AD, 5-HT3AE, and the homomeric 5-HT3A receptor.[9] N-terminal glycosylation of receptor subunits is critical for subunit assembly and plasma membrane trafficking.[10]
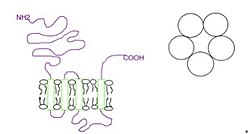
The subunits surround a central ion channel in a pseudo-symmetric manner (Fig.1). Each subunit comprises an extracellular N-terminal domain which comprises the orthosteric ligand-binding site; a transmembrane domain consisting of four interconnected alpha helices (M1-M4), with the extracellular M2-M3 loop involved in the gating mechanism; a large cytoplasmic domain between M3 and M4 involved in receptor trafficking and regulation; and a short extracellular C-terminus (Fig.1).[1] Whereas extracellular domain is the site of action of agonists and competitive antagonists, the transmembrane domain contains the central ion pore, receptor gate, and principle selectivity filter that allows ions to cross the cell membrane.[2]
Human and mouse genes
[edit]The genes encoding human 5-HT3 receptors are located on chromosomes 11 (HTR3A, HTR3B) and 3 (HTR3C, HTR3D, HTR3E), so it appears that they have arisen from gene duplications. The genes HTR3A and HTR3B encode the 5-HT3A and 5-HT3B subunits and HTR3C, HTR3D and HTR3E encode the 5-HT3C, 5-HT3D and 5-HT3E subunits. HTR3C and HTR3E do not seem to form functional homomeric channels, but when co-expressed with HTR3A they form heteromeric complex with decreased or increased 5-HT efficacies. The pathophysiological role for these additional subunits has yet to be identified.[11]
The human 5-HT3A receptor gene is similar in structure to the mouse gene which has 9 exons and is spread over ~13 kb. Four of its introns are exactly in the same position as the introns in the homologous α7-acetylcholine receptor gene, clearly showing their evolutionary relationship.[12][13]

Expression. The 5-HT3C, 5-HT3D and 5-HT3E genes tend to show peripherally restricted pattern of expression, with high levels in the gut. In human duodenum and stomach, for example, 5-HT3C and 5-HT3E mRNA might be greater than for 5-HT3A and 5-HT3B.
Polymorphism. In patients treated with chemotherapeutic drugs, certain polymorphism of the HTR3B gene could predict successful antiemetic treatment. This could indicate that the 5-HTR3B receptor subunit could be used as biomarker of antiemetic drug efficacy.
Tissue distribution
[edit]The 5-HT3 receptor is expressed throughout the central and peripheral nervous systems and mediates a variety of physiological functions.[14] On a cellular level, it has been shown that postsynaptic 5-HT3 receptors mediate fast excitatory synaptic transmission in rat neocortical interneurons, amygdala, and hippocampus, and in ferret visual cortex.[15][16][17][18] 5-HT3 receptors are also present on presynaptic nerve terminals. There is some evidence for a role in modulation of neurotransmitter release,[19][20] but evidence is inconclusive.[21]
Effects
[edit]When the receptor is activated to open the ion channel by agonists, the following effects are observed:
- CNS: nausea and vomiting center in brain stem, anxiety,[22] as well as anticonvulsant[23] and pro-nociceptive activity.[24][25]
- PNS: neuronal excitation (in autonomic, nociceptive neurons), emesis.[22]
Agonists
[edit]Agonists for the receptor include:
- Cereulide
- 2-methyl-5-HT
- Alpha-Methyltryptamine
- Bufotenin
- Chlorophenylbiguanide[22]
- Ethanol
- Ibogaine
- Phenylbiguanide
- Quipazine
- RS-56812 – Potent and selective 5-HT3 partial agonist, 1000× selectivity over other serotonin receptors
- SR-57227
- Varenicline[26]
- YM-31636[27]
- S 21007[28] (SAR c.f. CGS-12066A)
Antagonists
[edit]Antagonists for the receptor (sorted by their respective therapeutic application) include:
- Antiemetics
- Gastroprokinetics
- Alosetron
- Batanopride
- Metoclopramide (high doses)
- Renzapride
- Zacopride
- M1, the major active metabolite of mosapride
- Antidepressants
- Antipsychotics
- Antimalarials
- Others
- 3-Tropanyl indole-3-carboxylate
- Cannabidiol (CBD)
- Delta-9-Tetrahydrocannabinol
- Lamotrigine (epilepsy and bipolar disorder)
- Memantine (Alzheimer's disease medication)
- Menthol[29]
- Thujone
Positive Allosteric Modulators
[edit]These agents are not agonists at the receptor, but increase the affinity or efficacy of the receptors for an agonist:
- Indole Derivatives
- 5-chloroindole[30]
- Small Organic Anaesthetics
Discovery
[edit]Identification of the 5-HT3 receptor did not take place until 1986, lacking selective pharmacological tools.[14] However, with the discovery that the 5-HT3 receptor plays a prominent role in chemotherapy- and radiotherapy-induced vomiting, and the concomitant development of selective 5-HT3 receptor antagonists to suppress these side effects aroused intense interest from the pharmaceutical industry[2][33] and therefore the identification of 5-HT3 receptors in cell lines and native tissues quickly followed.[14]
See also
[edit]References
[edit]- ^ a b c d Barnes NM, Hales TG, Lummis SC, Peters JA (January 2009). "The 5-HT3 receptor--the relationship between structure and function". Neuropharmacology. 56 (1): 273–284. doi:10.1016/j.neuropharm.2008.08.003. PMC 6485434. PMID 18761359.
- ^ a b c d Thompson AJ, Lummis SC (2006). "5-HT3 Receptors". Current Pharmaceutical Design. 12 (28): 3615–3630. doi:10.2174/138161206778522029. PMC 2664614. PMID 17073663.
- ^ Reeves DC, Lummis SC (2002). "The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel (review)". Molecular Membrane Biology. 19 (1): 11–26. doi:10.1080/09687680110110048. PMID 11989819. S2CID 36985954.
- ^ Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF (1999). "The 5-HT3B subunit is a major determinant of serotonin-receptor function". Nature. 397 (6717): 359–363. Bibcode:1999Natur.397..359D. doi:10.1038/16941. PMID 9950429. S2CID 4401851.
- ^ Dubin AE, Huvar R, D'Andrea MR, Pyati J, Zhu JY, Joy KC, Wilson SJ, Galindo JE, Glass CA, Luo L, Jackson MR, Lovenberg TW, Erlander MG (1999). "The pharmacological and functional characteristics of the serotonin 5-HT3A receptor are specifically modified by a 5-HT3B receptor subunit". J Biol Chem. 274 (43): 30799–30810. doi:10.1074/jbc.274.43.30799. PMID 10521471.
- ^ Monk SA, Desai K, Brady CA, Williams JM, Lin L, Princivalle A, Hope AG, Barnes NM (2001). "Generation of a selective 5-HT3B subunit-recognising polyclonal antibody; identification of immunoreactive cells in rat hippocampus". Neuropharmacology. 41 (8): 1013–1016. doi:10.1016/S0028-3908(01)00153-8. PMID 11747906. S2CID 10168401.
- ^ Boyd GW, Low P, Dunlop JI, Ward M, Vardy AW, Lambert JJ, Peters J, Conolly CN (2002). "Assembly and cell surface expression of homomeric and heteromeric 5-HT3 receptors: The role of oligomerisation and chaperone proteins". Mol Cell Neurosci. 21 (1): 38–50. doi:10.1006/mcne.2002.1160. PMID 12359150. S2CID 37832903.
- ^ Niesler B, Walstab J, Combrink S, Moeller D, Kapeller J, Rietdorf J, Boenisch H, Goethert M, Rappold G, Bruess M (2007). "Characterization of the Novel Human Serotonin Receptor Subunits 5-HT3C, 5- HT3D and 5-HT3E". Mol Pharmacol. 72 (Mar 28): 8–17. doi:10.1124/mol.106.032144. PMID 17392525. S2CID 40072549.
- ^ Niesler, Beate (February 2011). "5-HT3 receptors: potential of individual isoforms for personalised therapy". Current Opinion in Pharmacology. 11 (1): 81–86. doi:10.1016/j.coph.2011.01.011. PMID 21345729.
- ^ Quirk, Phillip L.; Rao, Suma; Roth, Bryan L.; Siegel, Ruth E. (2004-08-15). "Three putative N-glycosylation sites within the murine 5-HT3A receptor sequence affect plasma membrane targeting, ligand binding, and calcium influx in heterologous mammalian cells". Journal of Neuroscience Research. 77 (4): 498–506. doi:10.1002/jnr.20185. ISSN 0360-4012. PMID 15264219. S2CID 25811139.
- ^ Sanger GJ (September 2008). "5-hydroxytryptamine and the gastrointestinal tract: where next?". Trends in Pharmacological Sciences. 29 (9): 465–471. doi:10.1016/j.tips.2008.06.008. PMID 19086255.
- ^ a b c Uetz, P; Abdelatty, F; Villarroel, A; Rappold, G; Weiss, B; Koenen, M (1994). "Organisation of the murine 5-HT3 receptor gene and assignment to human chromosome 11". FEBS Letters. 339 (3): 302–306. Bibcode:1994FEBSL.339..302U. doi:10.1016/0014-5793(94)80435-4. PMID 8112471. S2CID 28979681.
- ^ Uetz, P. (1992) Das 5HT3-Rezeptorgen der Maus. Diploma Thesis, University of Heidelberg, 143 pp.
- ^ a b c Yakel, JL (2000). Endo, M; Kurachi, Y; Mishina, M (eds.). The 5-HT3 receptor channel: function, activation and regulation in Pharmacology of Ionic Channel Function: Activators and Inhibitors. Handbook of Experimental Pharmacology. Vol. 147. Berlin: Springer-Verlag. pp. 541–560. ISBN 3-540-66127-1.
- ^ Férézou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B (2002). "5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons". J Neurosci. 22 (17): 7389–7397. doi:10.1523/JNEUROSCI.22-17-07389.2002. PMC 6757992. PMID 12196560.
- ^ Kazuyoshi Kawa (1994). "Distribution and Functional Properties of 5HT3 Receptors in the Rat Hippocampus Dentate Gyrus". Journal of Neurophysiology. 71 (5): 1935–1947. doi:10.1152/jn.1994.71.5.1935. PMID 7520482.
- ^ Sugita S, Shen KZ, North RA (1992). "5-hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala". Neuron. 8 (1): 199–203. doi:10.1016/0896-6273(92)90121-S. PMID 1346089. S2CID 22554779.
- ^ Roerig B, Nelson DA, Katz LC (1992). "Fast synaptic signaling by nicotinic acetylcholine and serotonin 5-HT3 receptors in developing visual cortex". J Neurosci. 17 (21): 199–203. doi:10.1523/JNEUROSCI.17-21-08353.1997. PMC 6573745. PMID 9334409.
- ^ Rondé P, Nichols RA (1998). "High calcium permeability of serotonin 5-HT3 receptors on presynaptic nerve terminals from rat striatum". J Neurochem. 70 (3): 1094–1103. doi:10.1046/j.1471-4159.1998.70031094.x. PMID 9489730.
- ^ Rondé P, Nichols RA (1997). "5-HT3 receptors induce rises in cytosolic and nuclear calcium in NG108-15 cells via calcium-induced calcium release". Cell Calcium. 22 (5): 357–365. doi:10.1016/S0143-4160(97)90020-8. PMID 9448942.
- ^ van Hooft JA, Vijverberg HP (2000). "5-HT3 receptors and neurotransmitter release in the CNS: a nerve ending story?". Trends Neurosci. 23 (12): 605–610. doi:10.1016/S0166-2236(00)01662-3. hdl:1874/7465. PMID 11137150. S2CID 36074796.
- ^ a b c d e Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4., page 187.
- ^ Gholipour T, Ghasemi M, Riazi K, Ghaffarpour M, Dehpour AR (January 2010). "Seizure susceptibility alteration through 5-HT(3) receptor: modulation by nitric oxide". Seizure. 19 (1): 17–22. doi:10.1016/j.seizure.2009.10.006. PMID 19942458.
- ^ Patel, Ryan; Dickenson, Anthony H. (September 2018). "Modality selective roles of pro-nociceptive spinal 5-HT2A and 5-HT3 receptors in normal and neuropathic states". Neuropharmacology. 143: 29–37. doi:10.1016/j.neuropharm.2018.09.028. ISSN 0028-3908. PMC 6277848. PMID 30240783.
- ^ Suzuki, Rie; Rahman, Wahida; Rygh, Lars J; Webber, Mark; Hunt, Stephen P; Dickenson, Anthony H (October 2005). "Spinal-supraspinal serotonergic circuits regulating neuropathic pain and its treatment with gabapentin". Pain. 117 (3): 292–303. doi:10.1016/j.pain.2005.06.015. ISSN 0304-3959. PMID 16150546. S2CID 6060490.
- ^ Mineur YS, Picciotto MR (December 2010). "Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis". Trends Pharmacol. Sci. 31 (12): 580–586. doi:10.1016/j.tips.2010.09.004. PMC 2991594. PMID 20965579.
- ^ Imanishi, N.; Iwaoka, K.; Koshio, H.; Nagashima, S. Y.; Kazuta, K. I.; Ohta, M.; Sakamoto, S.; Ito, H.; Akuzawa, S.; Kiso, T.; Tsukamoto, S. I.; Mase, T. (2003). "New thiazole derivatives as potent and selective 5-hydroxytriptamine 3 (5-HT3) receptor agonists for the treatment of constipation". Bioorganic & Medicinal Chemistry. 11 (7): 1493–1502. doi:10.1016/S0968-0896(02)00557-6. PMID 12628674.
- ^ Delagrange, Philippe; Emerit, M.Boris; Merahi, Nacera; Abraham, Christine; Morain, Philippe; Rault, Sylvain; Renard, Pierre; Pfeiffer, Bruno; Guardiola-Lemaître, Béatrice; Hamon, Michel (1996). "Interaction of S 21007 with 5-HT3 receptors. In vitro and in vivo characterization". European Journal of Pharmacology. 316 (2–3): 195–203. doi:10.1016/S0014-2999(96)00680-2. ISSN 0014-2999. PMID 8982686.
- ^ Ashoor, A.; Nordman, J.; Veltri, D.; Susan Yang, K. -H.; Shuba, Y.; Al Kury, L.; Sadek, B.; Howarth, F. C.; Shehu, A.; Kabbani, N.; Oz, M. (2013). "Menthol Inhibits 5-Ht3 Receptor-Mediated Currents". Journal of Pharmacology and Experimental Therapeutics. 347 (2): 398–409. doi:10.1124/jpet.113.203976. PMID 23965380. S2CID 111928.
- ^ Newman, A. S.; Batis, N; Grafton, G; Caputo, F; Brady, C. A.; Lambert, J. J.; Peters, J. A.; Gordon, J; Brain, K. L.; Powell, A. D.; Barnes, N. M. (2013). "5-Chloroindole: A potent allosteric modulator of the 5-HT3 receptor". British Journal of Pharmacology. 169 (6): 1228–1238. doi:10.1111/bph.12213. PMC 3831704. PMID 23594147.
- ^ Davies, Paul A (2011). "Allosteric modulation of the 5-HT3 receptor". Current Opinion in Pharmacology. 11 (1). Elsevier BV: 75–80. doi:10.1016/j.coph.2011.01.010. ISSN 1471-4892. PMC 3072441. PMID 21342788.
- ^ a b c Solt, Ken; Stevens, Renna J.; Davies, Paul A.; Raines, Douglas E. (2005-08-04). "General Anesthetic-Induced Channel Gating Enhancement of 5-Hydroxytryptamine Type 3 Receptors Depends on Receptor Subunit Composition". Journal of Pharmacology and Experimental Therapeutics. 315 (2). American Society for Pharmacology & Experimental Therapeutics (ASPET): 771–776. doi:10.1124/jpet.105.090621. ISSN 0022-3565. PMID 16081679. S2CID 22050514.
- ^ Thompson AJ, Lummis SC (2007). "The 5-HT3 receptor as a therapeutic target". Expert Opin Ther Targets. 11 (4): 527–540. doi:10.1517/14728222.11.4.527. PMC 1994432. PMID 17373882.
External links
[edit]- 5-HT3+Receptor at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
